The study throws light on the significance of bone morphogenetic factor protein signaling for the protection of a neural stem cell reservoir all through adult life. It may also help understanding the interplay between exercise, aging and neurogenesis. Experts observed that adult neural stem cells in the hippocampus generate new brain cells all through life. This specific area of the brain is mainly exposed to age-related deterioration. Shrinking of aging hippocampi is regulated by physical exercise. It also seems to enhance learning and memory among mature adults.
Senior author Fred H. Gage, Ph.D., a professor in the Laboratory for Genetics at the Salk Institute and the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Diseases shares, “This study provided us with very important insights into how adult stem cells are regulated. Going forward, we can start to tinker with this mechanism to understand how exercise influences the aging brain.”
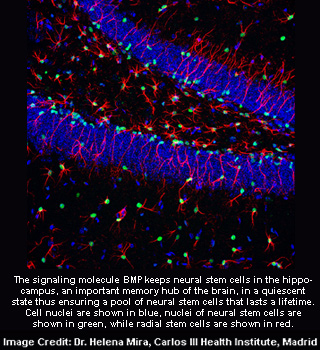
Neural stem cells in the hippocampus are kept by the signaling molecule BMP in a sluggish collection of neural stem cells that lasts a lifetime. Neurons pass through many stages during the process of neurogenesis. These stages include cell birth, fate determination, survival, integration, and acquisition of functional properties.
Co-first author Helena Mira, formerly a post-doc in the Gage laboratory and now an assistant professor in the Department of Cell Biology and Development at the Carlos III Health Institute in Madrid shares, “Each stage is driven by a complex interplay between intrinsic mechanisms and environmental cues. We already knew a lot about fate choice and differentiation, but it was unclear how neural stem cells decided to divide or not in the first place”.
Inactive neural stem cells reveal the BMP receptor 1A as a starting point. Experts identified the role of BMP signaling in controlling the proliferation of stem cells located in the hippocampus. They also examined BMP signaling, which is stimulated by the association of BMPs with their receptors. They observed they are inactive in most proliferating cells and active in non-dividing cells, including quiet stem cells and differentiated neurons. Mature neurons express BMP receptor 1B which will instigate further analyses.
Mira elucidates, “It tells you how finely this process is regulated. BMP ensures a sufficiently big population of quiescent stem cells that can feed into the system when called upon.”
Experiments with cultured neural stem cells assured that it was BMP that kept them quiet. When BMP was replaced with a protein known as Noggin BMP’s anti-proliferative effect was blocked. When they transferred Noggin directly into the brains of adult mice they revealed the same results. Inactive cells were moved from their slumber when noggin effectively interfered with BMP signaling. Neuron stem stems started fragmenting after one week and their offspring’s were on the verge of becoming neurons.
Gage reveals, “As we age, the number of new neurons declines but physical exercise brings that number back up. Our findings raise the possibility that the BMP signal becomes dominant over time, forcing neural stem cells deeper into quiescence and thus making it harder to generate new brain cells”.
Collection of active neural stem cells lowered when neural stem cells were stimulated to proliferate over a period of time. This underlined calm functions as a protective approach that counteracts stem cells exhaustion and burst of fragmenting cells which may lead to tumors.
These findings were published in the July 1 issue of Cell Stem Cell
