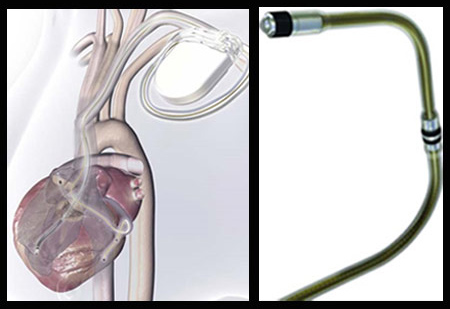
A novel device by Medtronic, Inc., the global pioneering provider of lifelong solutions for people suffering from chronic diseases has recently gained an approval from the U.S. FDA. Its Attain Ability left-heart lead is said to be a device which, along with cardiac resynchronization therapy (CRT) devices, may be useful in the treatment of heart failure patients.
This particular device is said to consist of the thinnest lead body of the left-heart lead models which are presently available. Thereby this latest development may prove to be helpful to the physicians in providing therapies right on the difficult-to-reach parts of the heart.
That is not all. This particular left-heart lead is stated to integrate a certain insulation material which has been developed by NASA Langley Research Center. This particular insulation material was believed to have been earlier assessed for use in high-performance engines, space applications, and harsh environments. It has been stated that this is seemingly the first time that a NASA material may have been utilized in implantable medical devices. This lead is said to have been created from an advanced aerospace resin called Langley Research Center’s Soluble Imide (LaRC-SI).
Pat Mackin, Senior Vice President at Medtronic says that, “Attain Ability is the latest innovation in our long-term strategy to provide physicians with a broad portfolio of leads and delivery systems to meet the unique needs of their patients. We’re proud to partner with NASA, another innovation leader, to provide physicians with a unique, technology-based solution to assist physicians with optimal lead placement in heart failure patients.â€
Seemingly the most difficult part of implanting CRT devices is said to be the part where a lead has to be piloted through the delicate and complex curves of the heart’s anatomy and placed on the ideal location of the left ventricle. More so in order to test the effective placement of this lead device a clinical trial was said to have been conducted on almost 200 patients. Seemingly physicians gained a 96.4 percent success rate in rightly placing the Attain Ability lead on the heart of the subjects.
Principal investigator of the clinical trial, Brian Ramza, MD, PhD, says that, “The unique design of the Attain Ability lead provides clinicians with a greater degree of flexibility in left ventricular lead placement. The lead provides the flexibility of multi-site pacing from small veins because of its design, therefore, truly having the potential to improve outcomes for patients with unique needs.â€
The Attain Ability lead is stated to be available in Europe, Canada, Australia and Japan along with the U.S. Further it has been stated that the entire Attain portfolio may be applicable with the company’s Vision 3D portfolio of wireless cardiac resynchronization therapy defibrillators.
