Certain diseases could be increasingly difficult to treat as antibiotic resistance spreads among bacteria. To address this growing problem, novel strategies that attack the bacteria are apparently needed. However, presently no such mechanism antibiotics are being developed. Gram-negative bacteria are specifically thought to be difficult to attack as they appear to possess a double wall.
Integrating an outer membrane surrounding the bacterial cell wall, which interferes with drug penetration, normally these cause stomach ulcers and a number of other serious diseases. With this new research, scientists intend at building a clearer picture of the mechanisms employed by Gram-negative bacteria to infect host’s cells and also how antibiotic resistance is spread by them.
‘Type IV secretion systems’, cellular ‘nanodevices’ are currently being analyzed by Professor Gabriel Waksman and colleagues at the Institute of Structural and Molecular Biology at Birkbeck and UCL (University College London). These supposedly behave like pumps and could span across the double membrane of the bacteria. While pumping antibiotic resistance genes into antibiotic-sensitive bacteria, they also appear to push out toxins into host cells. An opening in the protective covering of Gram-negative bacteria for new antibiotics to exploit could be delivered by this pump.
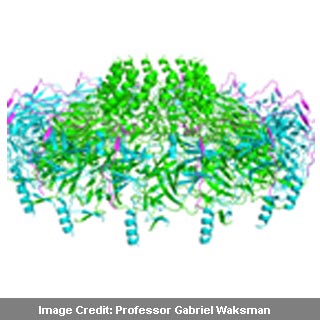
Earlier the structure of the core component of the pump had been described by Professor Waksman and colleagues. They had supposedly used a method called cryo-electron microscopy for this purpose. In the new paper, the experts now offer detail characterizations of the crystal structure of the outer membrane that is part of this complex. Unlike the earlier time, this time x-ray crystallography was used by the researchers. This claims to provide a higher resolution as against the cryo-electron microscopy, enabling identification and localization of each atom in the structure.
“X-ray crystallography gives us a much closer look at how this pump is working,” elucidates Professor Waksman. “It’s like examining a car: when you first look at a car, you see its shape, but might not understand how it works. It’s only by looking under the bonnet that you can see the engine and get a clearer idea of how it works. The big question for us is ‘how does this pump work?’ and that’s what we’re looking at now. By taking the pump apart and seeing how its engine works, we open up the way to ‘throwing a spanner in the works’ and stopping the pump working.”
If the experts associated with this research are to be believed then the pump’s component is the largest outer-membrane complex for which the structure is known at high resolution. This is apparently true even though the pump’s component is just a ten-thousandth of the width of a human hair. It appears to offer the first look of a channel that may be formed by more then one protein.
Membrane channels that are basic parts of the pump apparently allow the entry of toxins through the pump. Formed from proteins, these channels are incorporated in the membrane. Larger pumps like type IV secretion systems may include channels that are complex due to inclusion of many proteins. Three proteins with each present in fourteen copies appear to make up new structure. A first brief look at the inner workings of the machinery may be delivered by the distinct complexity of the arrangements of the proteins.
These findings have been published in a paper in the journal ‘Nature’.
