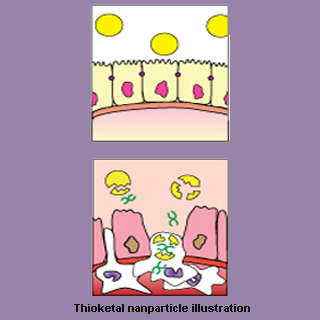
It is known that thioketal nanoparticles guard small interfering RNAs (siRNAs) from unsuitable environment of the gastrointestinal tract. These siRNAs may be targeted directly to the inflamed intestinal tissues for avoiding significant side effects caused if injected systemically. The thioketal nanoparticles were supposedly formulated from a new polymer known as poly-(1,4-phenyleneacetone dimethylene thioketal) (PPADT). Investigators claim to have engineered PPADT in a diameter of around 600 nanometers for optimal oral delivery.
Niren Murthy, an associate professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University, added, “The thioketal nanoparticles we designed are stable in both acids and bases and only break open to release the pieces of RNA in the presence of reactive oxygen species, which are found in and around inflamed tissue in the gastrointestinal tract of individuals with inflammatory bowel diseases.”
Experiments were initiated on mouse models of ulcerative colitis, which is a severe inflammatory bowel disease. Thioketal nanoparticles consisting siRNA were orally administered by the scientists. The siRNA is assumed to prevent an inflammation-promoting cytokine called tumor necrosis factor alpha (TNF-α). There is a possibility that nanoparticles migrate directly to the mouse colons where reactive oxygen species are produced in excess and cytokine production levels are reduced. Tissue samples from the colons treated with siRNA delivered by thioketal nanoparticles were thoroughly scrutinized by the researchers. It was concluded that the samples contained intact epitheliums, fingerlike ‘crypt’ structures and declined levels of inflammation. The findings reveal that colon may be safeguarded against ulcerative colitis.
Scott Wilson, a graduate student in the Georgia Tech School of Chemical and Biomolecular Engineering and lead investigator, remarked, “Since ulcerative colitis is restricted to the colon, these results confirm that the siRNA-loaded thioketal nanoparticles remain stable in non-inflamed regions of the gastrointestinal tract while targeting siRNA to inflamed intestinal tissues.”
Thioketal nanoparticles are possibly equipped with chemical and physical properties essential for facing obstacles of gastrointestinal fluids, intestinal mucosa and cellular barriers to give therapy for inflamed intestinal tissues. Investigations are underway for elevating the degradation rate of the nanoparticles and improving their reactivity with reactive oxygen species. Thioketal nanoparticles containing siRNA may have a cell toxicity profile very much alike the nanoparticles formulated from the FDA-approved material poly(lactic-co-glycolic acid) (PLGA). It is assumed that thioketal nanoparticles can help treat various gastrointestinal diseases related to intestinal inflammation, namely gastrointestinal cancers, inflammatory bowel diseases and viral infections.
The research was published on October 10 in the online edition of the journal Nature Materials.
