As part of the distinct research, experts blocked the function of YOD1 to identify its role. This included a manipulation that stopped the complete elimination of several misfolded model proteins. With this finding, scientists should be able to better comprehend this vital yet complex cellular process.
“It’s a great platform to dissect in the test tube how these complicated processes work,†mentioned Whitehead Member Hidde Ploegh. “The most exciting prospect would be that we could use this information to identify the channel through which these misfolded proteins are extracted from the ER, and that would potentially give us a new window on the process.â€
The researchers when analyzing human kidney cells discovered that YOD1 is an important part of a ring-shaped protein complex. This complex was found to pull misfolded proteins from a cellular compartment, called the endoplasmic reticulum (ER) into the surrounding cellular fluid. Practically all secreted proteins were found to be assembled and folded into their appropriate shapes in the ER. Proteins that were improperly folded could cling together, consequently clogging the ER. This, the experts say could be really harmful
“This is a life-threatening situation for the cell, so there is a constant quality control going on that gets rid of these misfolded proteins,†says Robert Ernst, a co-first author on the Molecular Cell paper and postdoctoral researcher in the lab of Whitehead Member Hidde Ploegh.
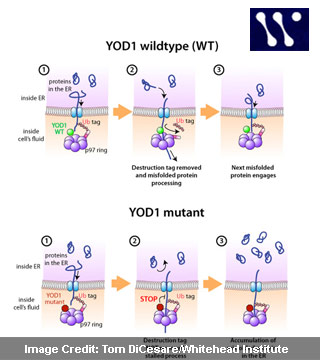
Active eradication of misfolded proteins from the ER may be a step in that quality control process. Emergence of a misfolded protein from the ER through a membrane channel may cause the cell to tag it for destruction. A specialized ring of proteins could be attracted to these tags. Reeling the misfolded protein from the ER through the center of the ring could be the task of these tags. Tagged misfolded proteins though could be too huge to efficiently pass through the ring’s center, the YOD1 protein seated atop the ring was found to eliminate the tags.
The cell appears to tag the protein for demolition again upon passage of the stripped misfolded protein through the protein ring. Nevertheless, this time around the tags seem to stay in place, shuttling the misfolded protein to a membrane-bound sack called the proteasome. The potent activity of the sack degrades proteins to their constituent parts.
In the course of the research, scientists also uncovered that the misfolded protein removal system entirely closes down at an early stage of removal when non-functioning YOD1 is substituted on the ring protein. Unable to keep tags at bay from the misfolded protein, the misfit YOD1 then jams the ring protein. This could leave the off-base protein stuck in both the ER channel and in the ring protein.
This research has been reported this month in Molecular Cell.
